
‘Display information on 7 common antibiotics’
Why in news?
- Central Drugs Standard Control Organisation (CDSCO) has now asked manufacturers that information of adverse reactions that were being reported from some commonly-used antibiotics be made available to the general public.
- The has been observed by Union Health Ministry’s pharmaceutical watchdog, the National Co-ordination Centre of the Pharmacovigilance Programme of India (PvPI).
More in news
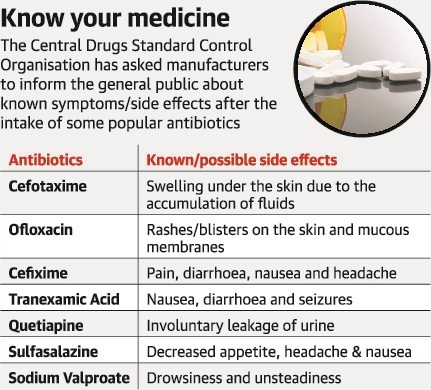
- What are the instructions?(1) CDSCO has written to drug manufacturers, to mention in leaflets inserted into drug packets or on promotional literature, information about the adverse reactions of these medicines.(2) All zonal and sub-zonal officers have been instructed to direct the manufacturers of these formulations to mention the additional reaction in the package insert or promotional literature of the drug.(3) All of the seven formulations have been instructed to warn patients of the “new” side effects.(4) These are:
- Antibiotics Cefotaxime.
- Ofloxacin and Cefixime.
- Tranexamic acid, used to control bleeding.
- Antipsychotic drug Quetiapine.
- Anti-rheumatoid drug Sulfasalazine.
- Anti-epileptic medicine Sodium Valproate.
Source
The hindu.

